Solutions - Common Ion Effect
MCAT General Chemistry
- Home
- »
- MCAT Masterclass
- »
- Chemical and Physical Foundations of Biological Systems
- »
- General Chemistry
- »
- Solutions – Common Ion Effect – MCAT General Chemistry
Sample MCAT Question
Which of the following will decrease the amount of dissolved iron (II) carbonate?
a) Adding iron (II) chloride to the solution
b) Removing iron ions from solution
c) Adding water to the solution
d) Adding more iron (II) carbonate to the solution
A is correct. Adding iron (II) chloride to the solution.
The chemical equation for the dissolution of iron (II) carbonate is: FeCO3 (s) --> Fe2+ (aq) + CO32- (aq). To decrease the amount of dissolved lead (II) chromate (which is essentially iron and carbonate ions), we need to shift the equilibrium to the left. When iron (II) chloride is added to solution, it will completely dissociate to form Fe2+ and Cl- ions. Therefore, the concentration of Fe2+ will increase, shifting the equilibrium for the dissolution of FeCO3 to the left. B is incorrect because removing iron ions will shift the reaction to the right. C is incorrect because adding water will not affect the equilibrium state at all. A is incorrect because adding more iron (II) carbonate will shift the reaction to the right, and if the solution is saturated, the added iron (II) carbonate will stay as a precipitant.
Solutions and the Common Ion Effect
When an electrolyte dissolves in solution, its associated ion pair dissociates into separate cations and anions. The equilibrium concentration of ions dissolved in solution is denoted by the solubility product constant (Ksp). However, what would happen if extra ions were added to a solution at equilibrium? This effect, the common ion effect, will be demonstrated through two examples.
Common Ion Effect - Example 1
A solution of dissolved CaCO3 is at equilibrium. What would happen to the amount of dissolved CaCO3 if CaCl2 is added to the solution?
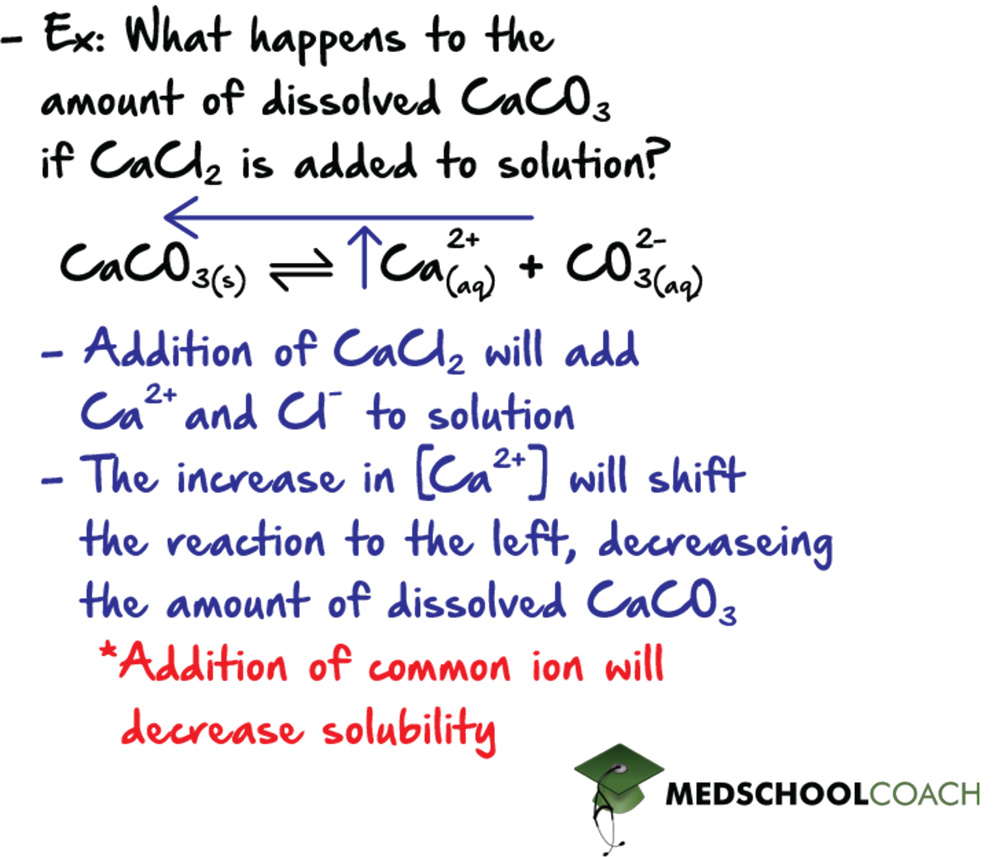
Applying Le Chatelier’s principle, a secondary equilibrium will begin, where CaCl2 will dissolve and dissociate to form calcium ions and chloride ions that will freely interact with solution. Both equilibria present in the solution share a common ion, calcium. The dissolution of CaCl2 increases the total amount of calcium ion present in solution. Remembering that the dissolution of CaCO3 is at equilibrium, an increase in the concentration of calcium will increase the ion product (Q) of CaCO3 past its Ksp. When Q > Ksp, excess solute precipitates out of solution. In this case, excess Ca2+ will combine with CO32-, forming solid CaCO3, which precipitates until Q = Ksp. The take home point here is that addition of a common ion will decrease solubility, potentially forcing some excess ion to form a precipitate.
Common Ion Effect - Example 2
A solution of dissolved CaCO3 is at equilibrium. What would happen to the amount of dissolved CaCO3 if HCl is added to the solution?

Recognizing that HCl is a strong acid and that CO3 is a weak base, we can predict that the two are going to undergo a neutralization reaction. As the hydrogen chloride protonates the bicarbonate, carbonate will be removed from solution. If ions are removed from equilibrium, we will reach a situation where Q < Ksp. When Q < Ksp, the reaction is unsaturated, and fewer ions are dissolved in solution than will be at equilibrium. To increase the ion product (Q), calcium carbonate will dissociate further into calcium and bicarbonate ions, until Q = Ksp. The take home point from this is example is that removal of a common ion will increase solubility.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.